ACLU: California ‘death penalty drug’ supply illegal
By Brian Rinker
The Guardsman
A shortage of the so-called “death penalty drug,” sodium thiopental, led the California Department of Corrections and Rehabilitation to search overseas for a new supplier, leaving death penalty opponents fuming about the shipment California received Jan. 20 from what they claim is an unregulated British source.
The CDCR received 521 grams of the drug a day before Hospira, the only Food and Drug Administration regulated manufacturer of the drug, had officially ceased its production.
“The CDCR acted unethically in obtaining sodium thiopental from their source in the U.K., Dream Pharma,” said Natasha Minsker, death penalty policy director at the American Civil Liberties Union of Northern California. “The source is questionable — a one-person drug shop operating out the back of a driving school somewhere in the U.K. It is unregulated and unapproved.”
Minsker said the impact of the sodium thiopental shortage started affecting California last summer and set off a scramble to find more of the drug.
The ACLU posted emails and documents on its website subpoenaed by the California Supreme Court detailing how the CDRC found its supply of the drug.
The CDCR got in touch with Arizona, which had found a supplier in the U.K., and through that source, ordered their own sodium thiopental, according to the emails.
The shipment was manufactured by a company in Britain called Archimedes Pharma, but was ordered from a different British supplier, CDCR Deputy Press Secretary Terry Thornton said.
The ACLU believes California’s supply of sodium thiopental is illegal because of who supplied it and how it was obtained. The U.K. has since stopped all exports of the drug.
“We would not be following state law if we didn’t try and obtain sodium thiopental,” Thornton said. “We are mandated by state law. We worked with the DEA, U.S. Customs and the FDA. The shipment was FDA approved.”
“No, it is not,” Stefanie Faucher, associate director of Death Penalty Focus said. “The FDA didn’t block the shipment, but that is very different than approving it.”
According to the FDA, they do not regulate products imported for the purpose of lethal injection. They consider sodium thiopental an unapproved, but medically necessary drug.
Hospira’s sodium thiopental, trademarked as Pentothal, was regulated by the FDA for medical purposes only.
“The FDA is choosing not to enforce the law,” Minsker said. “They’re required by Congress to approve the drugs imported into the U.S. If the FDA doesn’t regulate these drugs, no one will.”
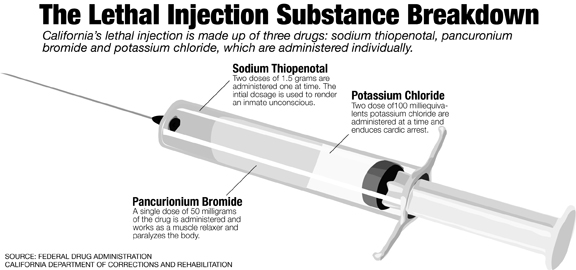
Sodium thiopental, a barbiturate responsible for the absence of pain during execution, is one of three drugs ensuring the lethal injection process in California to be humane. It is administered in two doses of 1.5 grams each and a back up injection of 2.5 grams. If the the drug is not refrigerated properly, problems could arise.
Minsker said the other two drugs used for lethal injection are known to cause horrible pain.
California law prohibits any change in the already approved lethal injection process without going through the Administrative Procedures Act, which requires the public to have an opportunity to participate in the adoption of state regulations.
“Whether California is going to accept another drug and change their protocol is up in the air right now,” Faucher said. “Right now the CDCR has a massive amount of sodium thiopental.”
With no known domestic suppliers of sodium thiopental remaining and no long-lasting suppliers emerging overseas, sodium thiopental might be difficult to find. But Thornton chose not to speculate.
“That’s way, way down the line,” Thornton said. “I have no way of knowing what will happen in the next five minutes. Do you?”
[like]

Comments are closed.